

Monosaccharides combine via the glycosidic bonds to form larger molecules such as disaccharides, trisaccharides, and polysaccharides. It shows that the number of water molecules in a monosaccharide is equal to the number of carbon atoms in it. Monosaccharides have general formula (CH 2O) x where x=number of carbon atoms. Hexoses include glucose, fructose, and galactose.īiologically important monosaccharides are trioses, pentoses, and hexoses.Tetroses such as erythrose and erythrulose.Trioses such as glyceraldehyde and dihydroxyacetone.Typesīased on the number of carbon atoms in their structure, monosaccharides have following types All the carbon atoms in a monosaccharide are attached to a hydroxyl group, except the one atom which is a part of either an aldehydic group or a ketonic group. This means that they have multiple hydroxyl groups(-OH) and have either an aldehyde group(-CHO) or a ketonic group (-CO-) in their structure. Some of them can have a ring structure.Ĭhemically, all the monosaccharides are either polyhydroxy aldehydes or ketones.They are polyhydroxy aldehydes or ketones.They contain only one molecule of sugar.Monosaccharides are known to have the following properties Monosaccharides undergo chemical combinations to form complex carbohydrate molecules such as starch, cellulose, and glycogen.

These are the monomers or building blocks of complex carbohydrates. Natural monomers or biological monomers are further divided into four categories.Īll these monomers are discussed in detail below. They join together to form larger molecules that then result in the formation of complex structures of living beings.
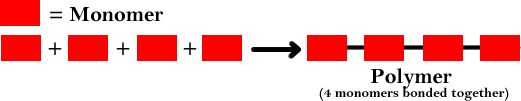
These synthetic monomers are then reacted together to form larger molecules used in industries for several beneficial purposes.Īs mentioned earlier, natural monomers are the bio-molecules that already exist in nature and are the building blocks of life on earth. Synthetic monomers are artificially made by combining different atoms for the welfare of mankind.
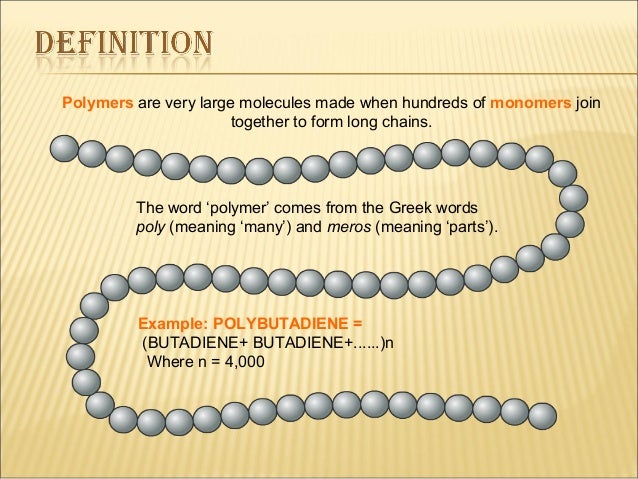
These molecules are responsible for all forms of life on our planet.


 0 kommentar(er)
0 kommentar(er)
